Introduction
For most patients (pts), multiple myeloma (MM) remains an incurable disease. Osteolytic lesions (OL) are present in 80% of pts at diagnosis and skeletal-related events are the most common cause for symptomatic relapse. Hence, myeloma bone disease (MBD) is a major cause of morbidity and mortality especially among pts with relapsed/refractory MM (RRMM).
B cell maturation antigen (BCMA) targeted chimeric antigen receptor (CAR) T cells have revolutionized treatment of patients with RRMM. While remission rates are high and pts experience long-standing treatment free intervals after CAR T cell treatment, little is known about its effects on MBD and bone turnover. The latter can be determined in plasma by measuring markers of bone formation and resorption. We aimed to analyze the longitudinal development of bone turnover during and after anti-BCMA CAR T cell treatment in RRMM pts.
Methods
We analyzed 28 consecutive pts with RRMM who underwent treatment with either Idecabtagene-Vicleucel (n=27) or Ciltacabtagen-Autoleucel (n=1) at our center between December 2021 and February 2023. Median age at infusion was 63 (range 39-75) years. 57% of pts were male. All pts were triple-class exposed, 21% had penta-class refractory disease. Status prior to CAR T cell infusion was complete remission (CR, 11%), partial remission (PR, 21%), very good PR (14%), stable disease (4%), or progressive disease (PD, 50%).
The impact of CAR T cell treatment on bone turnover was assessed by collecting plasma samples on the day of T cell apheresis, as well as days (d) 30 and 100 after CAR T cell infusion. Samples were analyzed by ELISA to evaluate plasma levels of bone formation marker Osteocalcin (OCN), bone resorption markers Tartrate-resistant acid phosphatase 5b (TRAcP5b) and fibroblast growth factor 23 (FGF23) as well as BCMA as a marker for the targeted myeloma cells. Median levels were compared between subgroups by Wilcoxon tests. Remission status after CAR T cell therapy was first evaluated at d 30 according to International Myeloma Working Group consensus criteria. Median follow-up was 6 months.
Results
Of the 28 pts treated with anti-BCMA CAR T cells, 64% achieved at least a PR (responders), while 36% had PD shortly (30 d) after. Within 14 d after infusion, 82% developed a cytokine release syndrome (CRS, °I or II), in 30% of which Tocilizumab was applied. Only 1 pt developed immune effector cell-associated neurotoxicity syndrome.
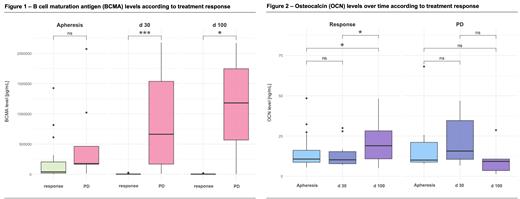
In responders, BCMA levels in plasma decreased significantly between apheresis and d 30 ( P<.01) and between apheresis and d 100 ( P<.01), while pts with PD showed no significant change in BCMA levels at the same timepoints ( P=.6 and P=.8). Furthermore, BCMA levels were significantly higher in pts with PD at d 30 ( P<.01) and d 100 ( P=.02), compared to responders (Figure 1).
Regarding bone resorption markers, TRAcP5b levels were significantly increased in responders ( P=.01) but not pts with PD ( P=.56) on d 30 after apheresis. Furthermore, we determined a significant increase of intact FGF23 between apheresis and d 30 ( P<.01) and a subsequent significant decrease of intact FGF23 ( P=.02) between d 30 and d 100 in responders. Since FGF23 and interleukin-6 (IL-6) are physiologically dependent, the observed FGF23 dynamics are likely to be an effect of increasing IL-6 in the context of CRS. Aptly, pts who received Tocilizumab because of full-blown CRS had higher intact FGF23 levels at d 30 ( P=.02) compared to pts without Tocilizumab treatment.
The bone formation marker OCN showed a significant increase in responders between apheresis and d 100 ( P=.01), as well as between d30 and d100 ( P=.02), while there were no significant changes in OCN levels over the time in pts with PD (Figure 2).
Conclusion
Pts responding to treatment with anti-BCMA CAR T cells, showed an increased bone turnover shortly after infusion. The increase of bone resorption marker levels appeared to be a short-term trend and could possibly be related to inflammation and high interleukin levels during the first days after infusion. However, we also saw a steady increase of bone formation marker OCN, which remained significant till d 100 in responders. Our data implies, that response to anti-BCMA CAR T cells is accompanied by increasing bone formation. Further analyses are needed to evaluate, whether this treatment can have positive long-term effects on MBD.
Disclosures
Ussmann:Sanofi: Other: former travel support. Kloetzer:Janssen: Honoraria. Heyn:Janssen: Honoraria. Herling:Abbvie, Beigene, Janssen, Stemline, Takeda: Consultancy; Mundipharma EDO, Janpix, Novartis, Roche: Research Funding. Jentzsch:Novartis: Honoraria; Blueprint Medicine: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; BMS: Honoraria; JAZZ: Honoraria. Platzbecker:Janssen Biotech: Consultancy, Research Funding; Curis: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; Fibrogen: Research Funding; BeiGene: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Roche: Research Funding; Geron: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Servier: Consultancy, Honoraria, Research Funding; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; AbbVie: Consultancy; BMS: Research Funding. Vucinic:BMS/Celgene: Consultancy, Honoraria, Other: Travel/Accommodations/Expenses; Amgen: Honoraria; Sobi: Honoraria, Other: Travel/Accommodations/Expenses; Takeda: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Abbvie: Honoraria; Gilead/Kite: Consultancy, Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Novartis: Consultancy, Honoraria. Merz:AMGEN, TAKEDA, BMS, JANSSEN, STEMLINE, ROCHE: Honoraria.